Mabaadi'da Thermodynamic
Si loo fahmo, qaab fudud, adduunyada balaaran ee qalafsan ee Thermodynamics, waxaa lagugula talinayaa inaad talaabo talaabo uqaado adigoo ka bilaabaya dib u eegista ereyada aasaasiga ah, hordhaca mabaadi'da heer-kulka, kadibna aad si qoto dheer u baraneyso sharciyada heer-kulyada, waxaa lagu muujiyaa xisaab ahaan iyo codsiyadeeda.
Iyada oo afarta sharci ee thermodynamics (eber sharciga, sharciga koowaad, sharciga labaad iyo sharciga saddexaad), waxaa lagu sharaxay sida wareejinta iyo isbadalka tamarta ee nidaamyada kaladuwan u shaqeeyaan; ahaanshaha aasaaska fahamka astaamaha badan ee dabiiciga ah.
Dib u eegista fikradaha aasaasiga ah
Waxaan kugu martiqaadeynaa inaad aragto maqaalka THERMODYNAMICS, waxa ay tahay iyo barnaamijyadeeda

Waad ku kabi kartaa macluumaadkan maqaalka Awoodda Sharciga Watt (Codsiyada - Jimicsiyada) Wixii hadda WAAN RAACAYNAA ...
Foomamka tamarta
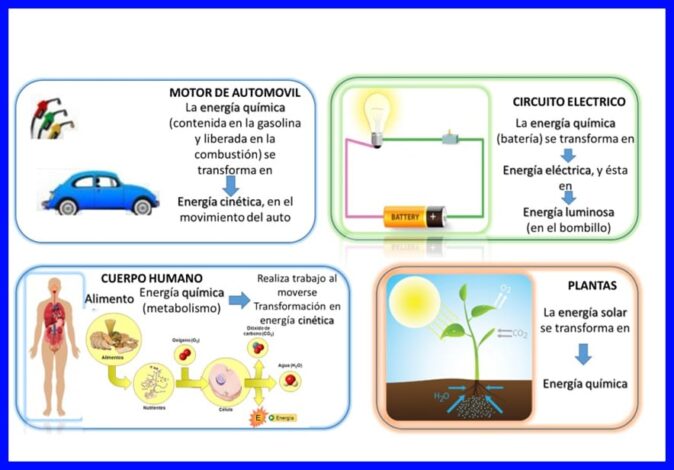
Tamarta, hantida ay leeyihiin meydadka si ay isu beddelaan iyagoo wax ka beddelaya xaaladdooda ama xaaladdooda, waxay ku timaaddaa qaabab badan, sida tamarta firfircoonida, tamarta iman karta iyo tamarta gudaha ee jirka. Fiiri sawirka 1aad.
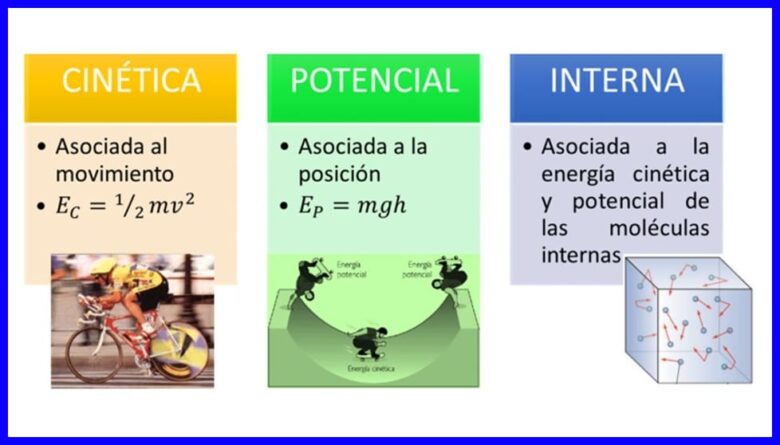
Shaqada
Waa wax soo saar xoog iyo barakac ah, oo labadaba hal jiho loogu wada cabbiray. Si loo xisaabiyo shaqada, waxaa loo isticmaalaa qaybta xoogga ee barbar socota barokaca shayga. Shaqada waxaa lagu cabiraa Nm, Joule (J), ft.lb-f, ama BTU. Fiiri sawirka 2aad.
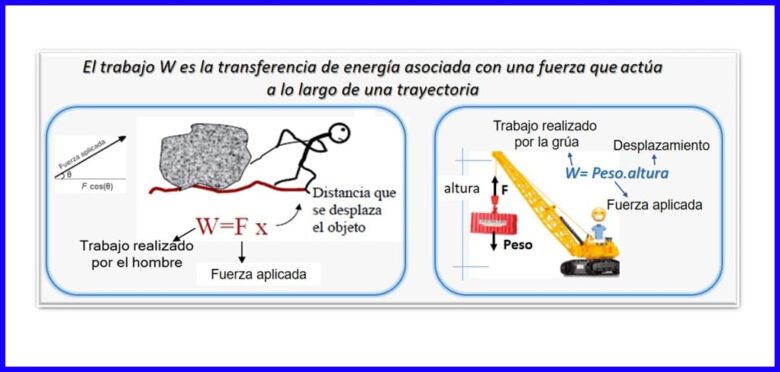
Kuleylka (Q)
Kala wareejinta tamarta kuleylka ah ee u dhaxeysa labo meydood oo ku jira heerkullo kala duwan, waxayna ku dhacdaa oo keliya dareenka ah in heerkulku hoos u dhacayo. Kuleylka waxaa lagu cabiraa Joule, BTU, pound-feet, ama kalooriyada. Fiiri sawirka 3.
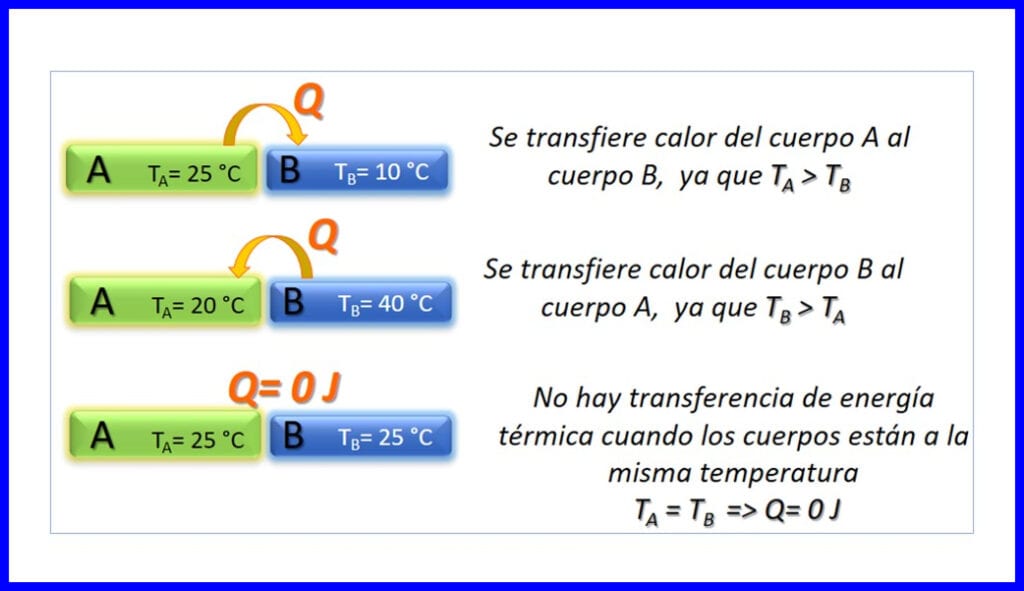
Mabaadi'da Thermodynamic
Sharciga Sero - Mabda'a Sero
Sharciga eber ee thermodynamics wuxuu sheegayaa in haddii laba shay, A iyo B, ay kujiraan isu dheelitirnaan heerkul ah oo midba midka kale leeyahay, isla markaana sheyga A uu la mid yahay sheyga seddexaad ee C, ka dibna sheyga B uu ku jiro isugeyn heerkul ah oo leh sheyga C. Isu dheelitirka heerkulka ayaa dhacaya markay laba ama in ka badan oo meyd ahi isku heer-kul yihiin. Fiiri sawirka 4aad.
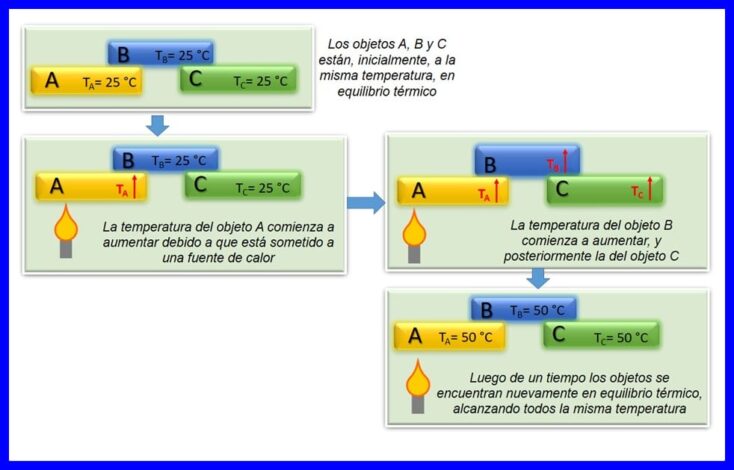
Sharcigan waxaa loo tixgeliyaa inuu yahay sharci aasaasi ah oo ku saabsan thermodynamics. Waxaa loo dhajiyay "Zero Law" 1935, tan iyo markii la soo saaray ka dib markii la sameeyay sharciyada koowaad iyo labaad ee thermodynamics.
1st Sharciga Thermodynamics (Mabda'a ilaalinta tamarta)
Bayaanka Sharciga Koowaad ee Thermodynamics:
Sharciga ugu horeeya ee thermodynamics, sidoo kale loo yaqaan mabda'a ilaalinta tamarta, wuxuu sheegayaa in tamarta aan la abuurin ama la dumin, kaliya waxaa loo beddelaa nooc kale oo tamar ah, ama waxaa looga wareejiyaa hal shay oo mid kale. Sidaa darteed wadarta guud ee tamarta koonku isma beddeleyso.
Sharciga ugu horeeya waxaa lagu fuliyaa “wax walboo”, tamarta waa la wareejiyaa oo si isdaba joog ah ayaa loo bedelaa, tusaale ahaan, aaladaha korontada qaarkood, sida qaswadayaasha iyo isku darka, tamarta korantada waxaa loo badalaa tamar farsamo iyo heer kul, jirka bini'aadamka waxaa loo bedelaa kiimikada Tamarta cuntada ee kujirta tamarta firfircoonida marka jirku socdo, ama tusaalooyin kale sida kuwa kujira jaantuska 5.
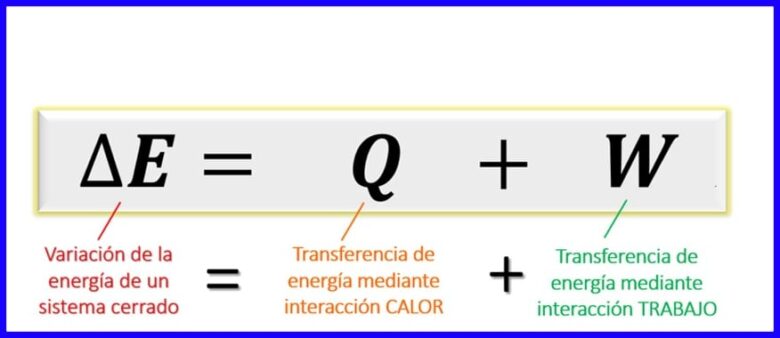
Isle'eg Sharciga Koowaad ee Thermodynamics:
Isleegga sharciga ugu horeeya ee mabaadi'da kuleylka (thermodynamic) wuxuu muujinayaa isku dheelitirka ay tahay inuu jiro inta udhaxeysa noocyada kaladuwan ee tamarta marka lajoogo. Maaddaama, nidaamyada xiran [1], is-weydaarsiga tamarta waxaa lagu bixin karaa oo keliya wareejinta kuleylka, ama shaqada la qabtay (nidaamka ama nidaamka) waxaa lagu caddeeyay in kala-duwanaanta tamarta ee nidaamku ay la mid tahay wadarta ku wareejinta tamarta kuleylka iyo shaqada. Fiiri sawirka 6.
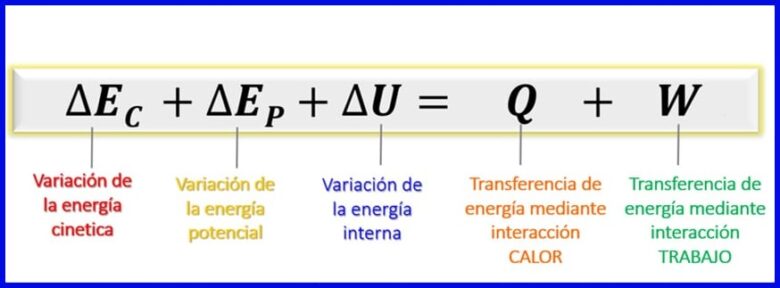
Marka la tixgeliyo in tamarta lagu tixgeliyey isku dheelitirka tamarta ay tahay tamar firfircoonida, tamarta iman karta iyo tamarta gudaha [1], isu dheelitirka tamarta ee nidaamyada xiran ayaa weli ah sida lagu muujiyey sawirka 7
- (c) Tamarta jirka, dhaqdhaqaaqa jirka darteed;
- (ep) Tamarta suurtagalka ah, ay ugu wacan tahay booska jirku kujiro cuf isjiidashada;
- (AMA) Tamarta gudaha, iyada oo ay ugu wacan tahay tabarucyada microscopic-ka ee firfircoonida iyo tamarta jir ahaaneed ee maaddooyinka gudaha ee jirka.
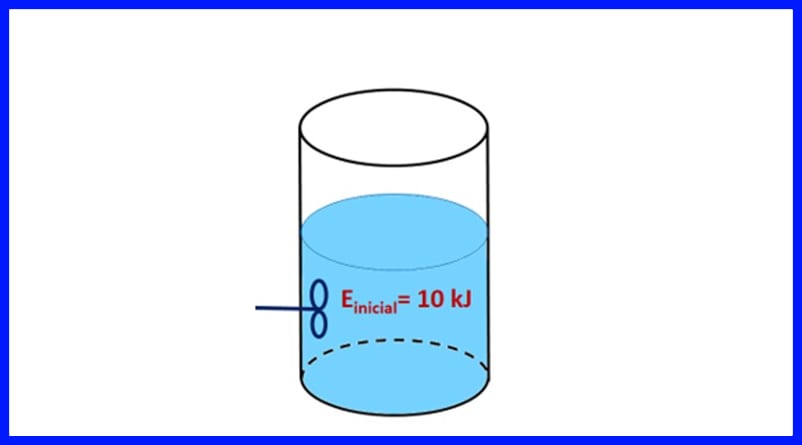
Layli 1
Weelka xiran ayaa ku jira walax, oo leh tamar bilow ah 10 kJ. Maaddada waxaa lagu walaaqay baytarro sameeya 500 J shaqo, halka isha kuleylka ay u gudbineyso 20 kJ oo kuleyl ah walaxda. Intaa waxaa dheer, 3kJ oo kuleyl ah ayaa loo sii daayaa hawada inta hawshu socoto. Go'aami tamarta ugu dambeysa ee walaxda. Fiiri sawirka 8.
Xalka:
Jaantuska 9aad waxaad arki kartaa kuleylka lagu daro isha kuleylka, taas oo loo arko inay tahay "togan" maaddaama ay kordhiso tamarta walaxda, kuleylka loo sii daayo hawada, diidmo maadaama ay yareyneyso tamarta walaxda, iyo shaqada korantada, taas oo kordhisay tamarta waxay qaadatay calaamad wanaagsan.
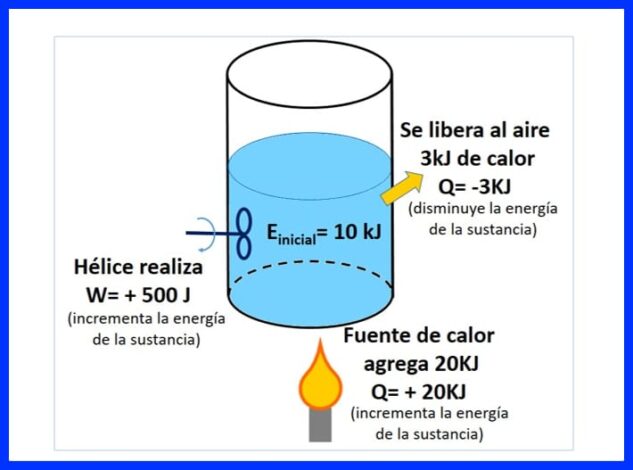
Jaantuska 10aad dheelitirka tamarta ayaa la soo bandhigay, sida ku xusan sharciga ugu horreeya ee thermodynamics iyo tamarta ugu dambeysa ee walaxda ayaa la helaa.

Sharciga labaad ee thermodynamics
Waxaa jira dhowr bayaan oo sharciga labaad ee thermodynamics: Bayaanka Planck-Kelvin, Clausius, Carnot. Mid kasta oo ka mid ahi wuxuu muujinayaa dhinac ka duwan sharciga labaad. Guud ahaan sharciga labaad ee thermodynamics wuxuu soo bandhigayaa:
- Jihada geedi socodka kuleylka, dib u noqoshada ifafaalaha jirka.
- Waxtarka mashiinnada kuleylka.
- Gali guriga "entropy".
Tilmaamaha hababka kuleylka:
Dabeecadda si iskiis ah, tamarta ayaa u socota ama looga wareejiyaa gobolka tamarta ugu sarreeya loona wareejiyo gobolka tamarta ugu yar. Kulaylku wuxuu ka yimaadaa meydadka kulul ilaa meydadka qabow ee dhanka kale uma socdo. Fiiri sawirka 11.

Karti ama waxqabadka kuleylka:
Marka loo eego sharciga ugu horeeya ee thermodynamics, tamarta lama abuurin mana burburin, laakiin waa la badali karaa ama la wareejin karaa. Laakiin dhammaan wareejinta tamarta ama beddelka qaddar ka mid ahi faa'iido ma leh in la qabto shaqo. Marka tamarta la wareejiyo ama la beddelo, qayb ka mid ah tamarta bilowga ah waxaa loo sii daayaa sida tamarta kuleylka: tamarta ayaa hoos u dhacda, waxay lumisaa tayada.
Isbadal kasta oo tamar ah, qadarka tamarta la helo ayaa had iyo jeer ka yar tamarta la bixiyo. Waxtarka kuleylka waa xaddiga kuleylka isha laga helo ee shaqada loo beddelayo, saamiga u dhexeeya tamarta waxtarka leh ee la helay iyo tamarta la bixiyay ee isbeddelka. Fiiri sawirka 12.

Mashiinka kuleylka ama Mashiinka kuleylka:
Mashiinka kuleylka waa aalad qayb ahaan u badaleysa kuleylka shaqo ama tamar farsamo, tan waxay u baahan tahay il siisa kuleylka heerkulka sare.
Mashiinnada kuleylka waxaa loo isticmaalaa walax sida uumiga biyaha, hawo ama shidaal. Maaddada waxay ku dhacdaa taxane ah is-beddelka kuleylka ee habka wareegga, si mashiinku u shaqeyn karo si joogto ah.
Layli 2
Mashiinka gawaarida xamuulka wuxuu soo saaraa kuleylka gubashada shidaalka. Wareeg kasta oo mishiinka ah, kuleylka 5 kJ waxaa loo beddelaa 1kJ oo ah shaqo farsamo. Waa maxay waxtarka mootada? Immisa kuleyl ayaa loo sii daayaa wareeg kasta oo mashiinka ah? Fiiri sawirka 13
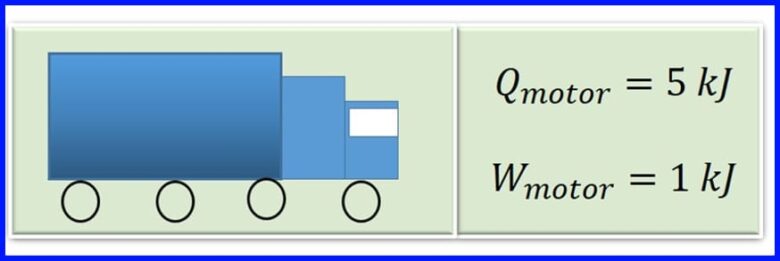
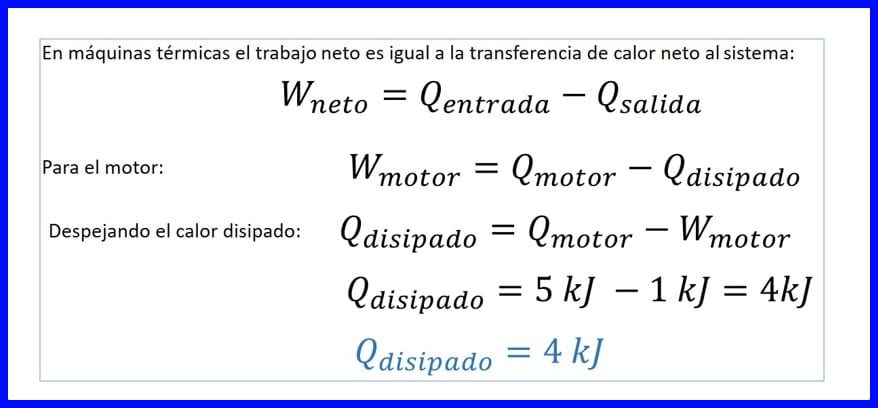
Xalka:

Si loo go'aamiyo kuleylka la sii daayay, waxaa loo maleynayaa in mashiinnada kuleylka ah shaqada saafiga ah ay la mid tahay wareejinta kuleylka saafiga ah ee nidaamka. Fiiri sawirka 14.
Entropy:
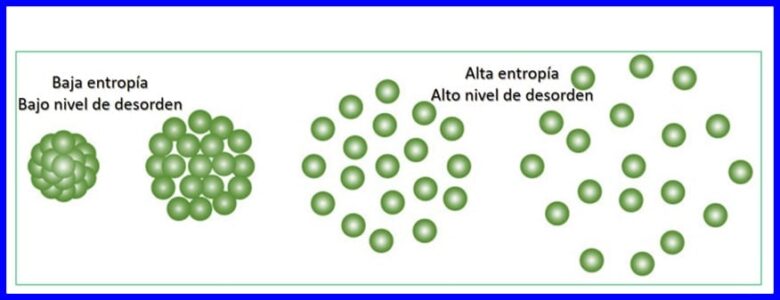
Entropy waa heerka kala sooc la'aanta ama khalkhalka nidaamka. Entropy waxay suurtogal ka dhigeysaa in la cabiro qeybta tamarta ee aan loo adeegsan karin in lagu soo saaro shaqo, taasi waa, waxay suurta gal ka dhigeysaa in la qiyaaso dib u noqoshada nidaamka thermodynamic.
Kala wareejin kasta oo tamar ah oo dhacdaa waxay kordhisaa entropy-ka koonkan waxayna yareysaa qadarka tamarta la isticmaali karo ee la heli karo si shaqo loogu qabto Nidaam kasta oo heer-kul-socod ah wuxuu ku socon doonaa jihada kordhinaysa wadarta entropy ee koonka. Fiiri sawirka 15.
3aad Sharciga Thermodynamics
Sharciga saddexaad ee Thermodynamics ama Nerst Postulate
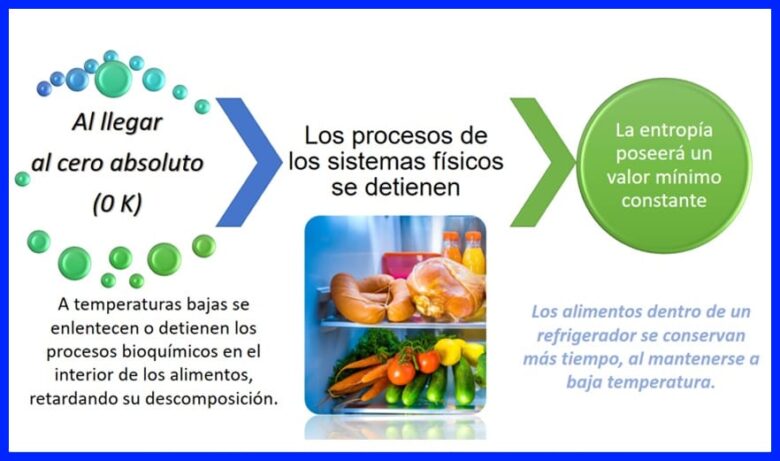
Sharciga saddexaad ee thermodynamics wuxuu la xiriiraa heerkulka iyo qaboojinta. Waxay sheegaysaa in qaab-dhismeedka eber dhammaystiran yahay mid joogto ah. Fiiri sawirka 16.
Eber Absolute waa heerkulka ugu hooseeya ee hooseeya oo aan hadda ka dib cabir hoose lahayn, waa kan ugu qabow ee jirku noqon karo. Eber dhammaystiran waa 0 K, oo u dhiganta -273,15 ºC.
Gabagabo
Waxaa jira afar mabda 'thermodynamic. Mabda 'eber ahaan waxaa loo aasaasay in isu-dheelitirnaanta kuleylka ay dhacdo marka laba ama in ka badan ay isku heer-kul yihiin.
Sharciga ugu horeeya ee thermodynamics wuxuu ka hadlayaa ilaalinta tamarta inta udhaxeysa howlaha, halka sharciga labaad ee thermodynamics uu ka hadlayo jiheynta laga bilaabo ugu hooseysa iyo tan ugu sarreysa, iyo waxtarka ama waxqabadka matoorada kuleylka ee kuleylka u beddelaya shaqada.
Sharciga seddexaad ee thermodynamics wuxuu laxiriiraa heerkulka iyo qaboojinta, wuxuu sheegayaa in soojiidashada nidaamka eber dhammaystiran yahay mid joogto ah.